Principal investigator: Piotr Bałczewski
Scientific goal of the project
Heteroatom variants of the Friedel-Crafts/Bradsher (F-C/B) cyclization, which would allow a deliberate introduction of at least one exo-heteroatom and high ring substitution in one time, on the fused (hetero)aromatic framework are very poorly recognized. For this purpose, in the synthetic stage I of the project, we would like to elaborate the phospho-F-C/B cyclization, which is a new member of the heteroatom family of reactions based on the electrophilic aromatic substitution of ortho-X,X-acetal diarylmethanes 2 (X = O, S) substituted with PIII-V-containing groups under acidic conditions. Why it is important? Because through these structure modifications, we can influence properties of the obtained P-(hetero)acenes 1. Therefore, in the stage II, we would like to test impact of the phosphorus presence in the obtained fluorophoric P-(hetero)acene 1 products on their thermal and photophysical properties, and photochemical stability under aerobic and anaerobic conditions at 254 and 365 nm, for the reasons given below (Scheme 1).
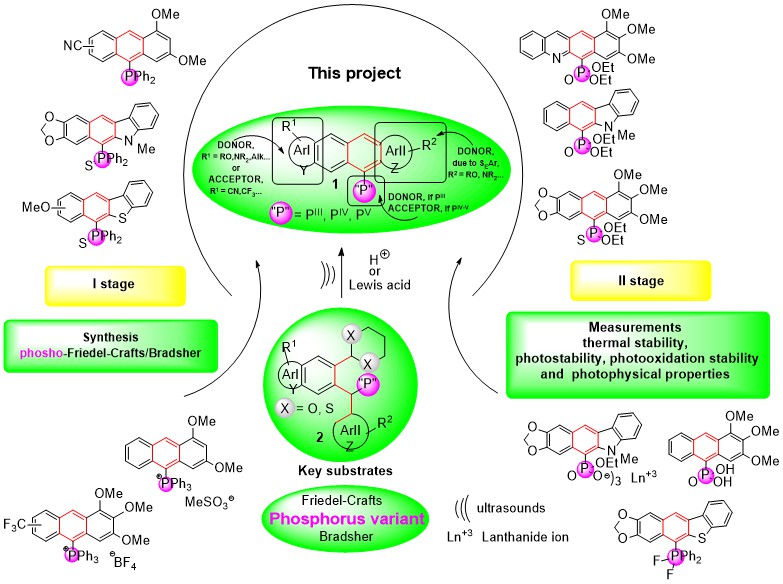
Scheme 1. Main goals of this project (stage I – synthesis and stage II – measurements).
Why phosphorus atom?
It would be difficult to find another heteroatom in the periodic table that would bring as many different benefits at the same time as phosphorus atom. Let’s enumerate them: 1) a varied coordination number in PIII-V compounds, as a consequence of pyramidal p3, tetrahedral sp3 or trigonal bipyramidal sp3d molecular geometry of P atom, 2) an ability to change the degree of coordination from lower to higher and vice versa, by conversions of PIII to IVP=X, IVP+ and VP-F compounds, and reduction of the latter back to PIII compounds, 3) an ability to form neutral (PIII, IVP=X, VP) and ionic (IVP+) compounds, 4) an ability of IIIP and IVP phosphonic acids to form complexes and salts with lanthanides that are vital for applications in OLEDs and organic lasers, 5) a possibility to introduce onto (hetero)aromatic moiety both electron-acceptor AAr and electron-donor DAr groups which operate together with P-containing groups of the gradually increasing AP character: IIIP(DP)>IVP=X (AP) (X = O, S)>VPR3F2 (AP)>IVP+ (AP). Moreover, 31P-NMR technique enables monitoring reactions progress, compounds purity and structural changes around phosphorus atom in the obtained P-(hetero)acenes 1. The easy change of the coordination number of phosphorus atom and modification of aromatic rings and ring substituents gives broad possibilities to influence the P-(hetero)acenes 1 properties, among other those, which are connected with light absorption and light emission phenomena.
Why P-(hetero)acenes?
There are many reasons. One of them is that organic molecular solids with a high degree of unsaturation, like P-(hetero)acenes 1, are semiconductors which may be used in organic photo and electroluminescent devices, phototherapies, photosensors, etc. A particular role in these devices is played by the compounds having donor (D) and acceptor (A) groups for a charge-transfer state, whose presence is an important factor influencing the increase in photophysical response of the investigated compounds. The majority of these compounds exhibit favorable p-type character, while a number of suitable n-type species with electron accepting chromophores is surprisingly low. Introduction of phosphorus atom at different oxidation states (III-IV) on the π-conjugated systems, guarantees both electron donor D (PIII) and especially electron acceptor A (IVP=X, IVP+, VP-F) properties which are beneficial for the synthesis of rare n-type semiconductors.