
Our research focuses on various aspects of extracellular vesicles (exosomes) secreted by human cells, and their roles in conditions like cancerous disease and inflammation. Extracellular vesicles are membrane-enclosed miniscule entities secreted by virtually all cells in human immune system, carrying signalling mediators affecting behaviour of immune cells, including lipids, nucleic acids and proteins. The process of exosome secretion, as well as the cargo they carry, is strictly regulated by the environmental conditions that the cells are subject to. Exchange of extracellular vesicles between cells constitutes an important mean of intercellular communication for coordination of immune responses in cancer and inflammatory diseases. We conduct studies both on primary cells and cancer cell lines
The main focus of our group is to study new regulatory mechanisms driving the secretion and load selection of extracellular vesicles. Currently, we want to understand how cancer cells utilize the secreted vesicles to inhibit immune responses of humans, we are using the mechanisms of intercellular communication used by cancer to create an element that supports anticancer therapy. Our goal is to modulate cancer cells to inhibit the secretion of exosomes and sensitize the cells to anticancer therapy.
Our research is supported both by the statutory funds for CMMS PAS and grants from the National Science Centre.
If you are interested in knowing more about our projects, or you would like to collaborate with us
CONTACT US AND JOIN OUR TEAM!
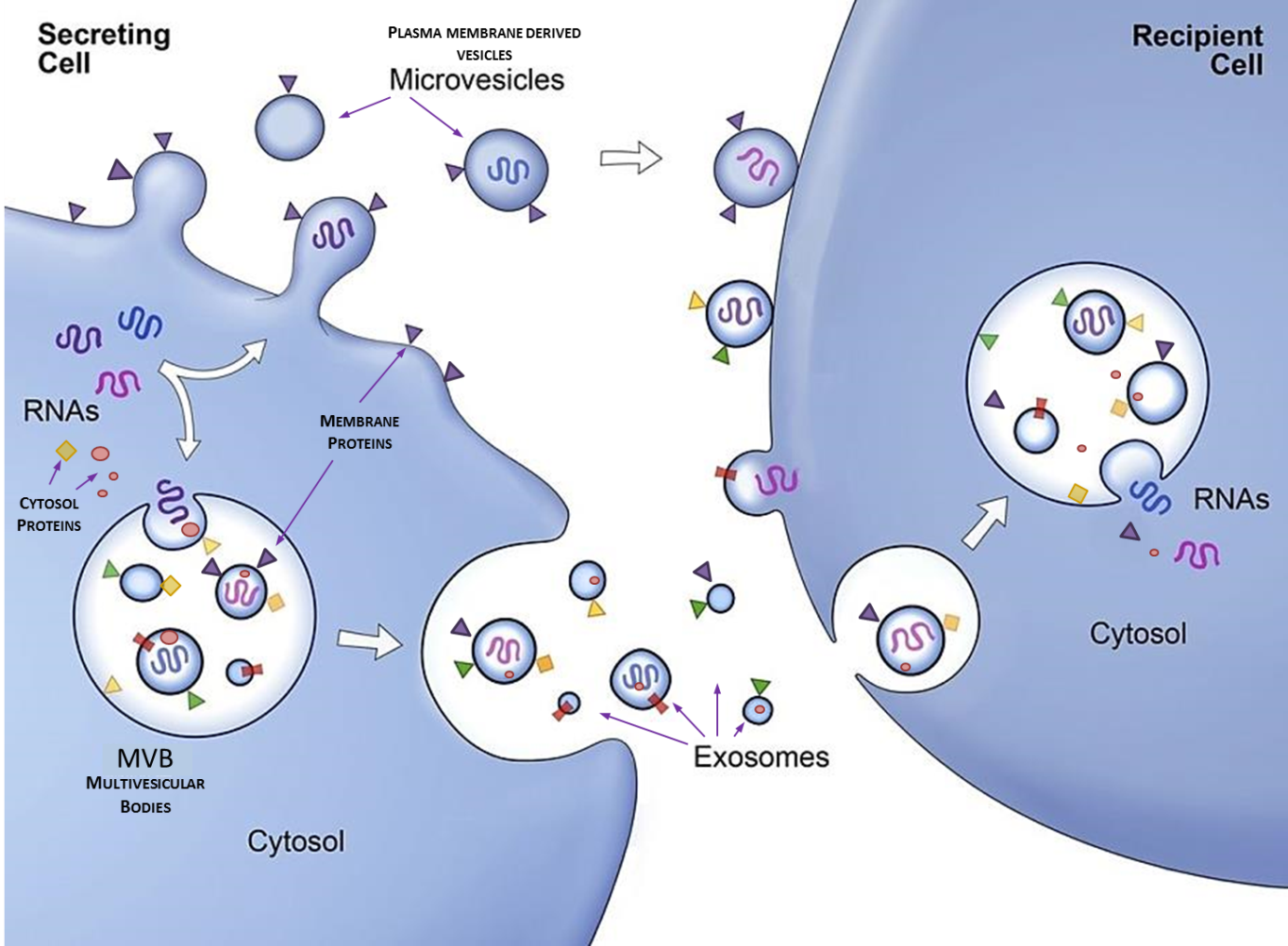

Current research projects:
Creation of immunosuppressive exosomes for the induction of antigen-specific tolerance – grant OPUS 11 (2016/21/B/NZ7/02747), 2017-2020, dr hab. Markus Düchler
Characterization of secretion, composition and immune properties of the extracellular vesicles released during NLRP3 inflammasome activation, with proteomics combined with bioinformatics approach. – grant SONATINA 2 (2018/28/C/NZ6/00069), 2018-2021, dr inż. Wojciech Cypryk
Modulation of exosome production to support anti-cancer treatment – grant OPUS 18 (2019/35/B/NZ7/03256), 2020-2023, dr hab. Markus Düchler
1. Melanoma-derived exosomes and the induction of antigen-specific immune tolerance.
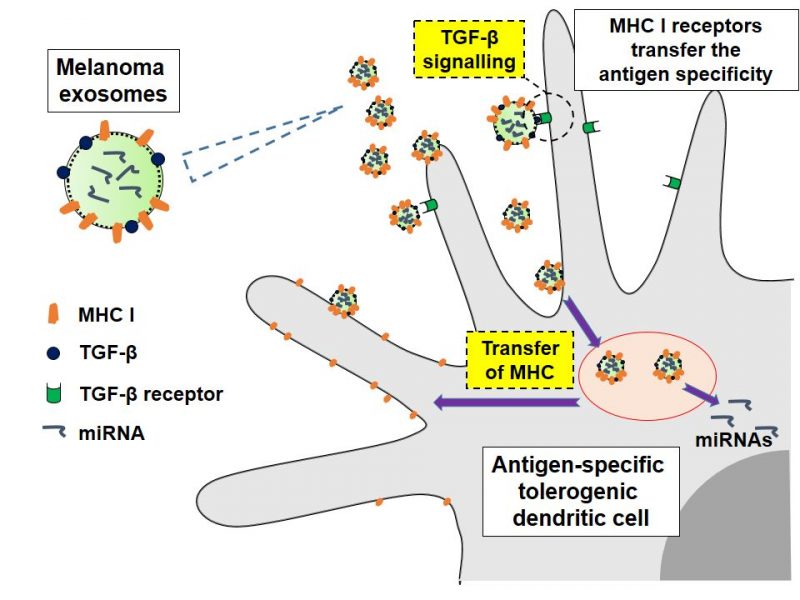
Inhibition of antitumor immune response is crucial for the survival and development of tumors. This phenomenon is however complex and dependent on seemingly different aspects in different tumors. Specifically – it is of antigen-specific nature – dependent on different antigens recognized by immune system on different cancer. Our studies focus on the function of small extracellular vesicles (exosomes) on immunosupression. These vesicles are decorated with major histocompatibility complexes (MHC) class I, which are able of presenting specific tumor cell-derived antigens and induce adaptive immune reponses, in a process of cross-dressing. Simultaneously these vesicles carry a cargo of proteins and microRNAs, which are able to suppress immune responses modulating the acceptor cell phenotype. Such process may lead to antigen-specific tolerance and inhibition of natural antitumor defense mechanisms. The results of our studies confirm that this indeed is the case.
2. Creating immunosuppressive exosomes
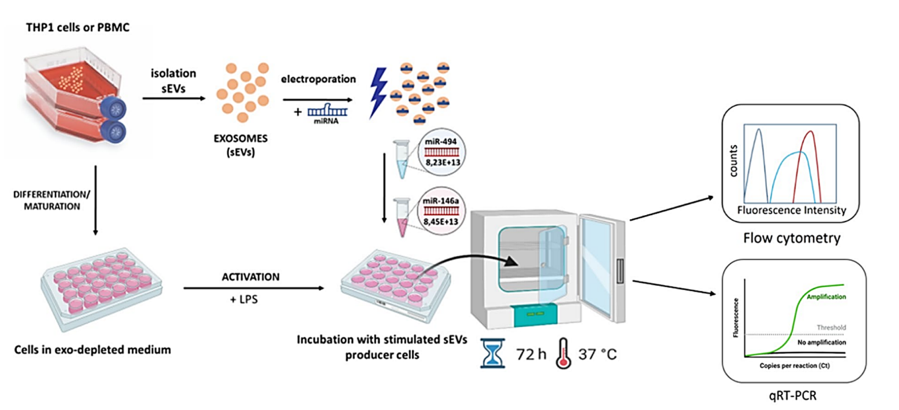
The abovementioned process of exchange of biological information via vesicular transport may facilitate and promote the survival of tumors in humans. Similar strategy may also be adopted to reverse this process – by loading exosomes with molecules that could inhibit the immunosuppressive effect. Such approach would be highly desired for example for inhibiting graft rejections, which happen in patients receiving organ transplants, who currently must be treated with high doses of immunosuppressive drugs that lower their immunity. Engineered extracellular vesicles equipped with immunosuppressive potential could be designed to act selectively – in an antigen-specific manner. Such solution would constitute an excellent therapeutic option treatment of autoimmune diseases and graft-versus-host disease.
Our studies provide the evidence that sEVs can be used to create tools for immunosuppression similar to the strategies used by tumour. Experiments show that simple manipulations of sEVs using immunosuppressive cargo like miRNA can lead to the inhibition of DC maturation by decreasing the expression of MHC molecules.
3. Extracellular vesicles signaling in inflammation
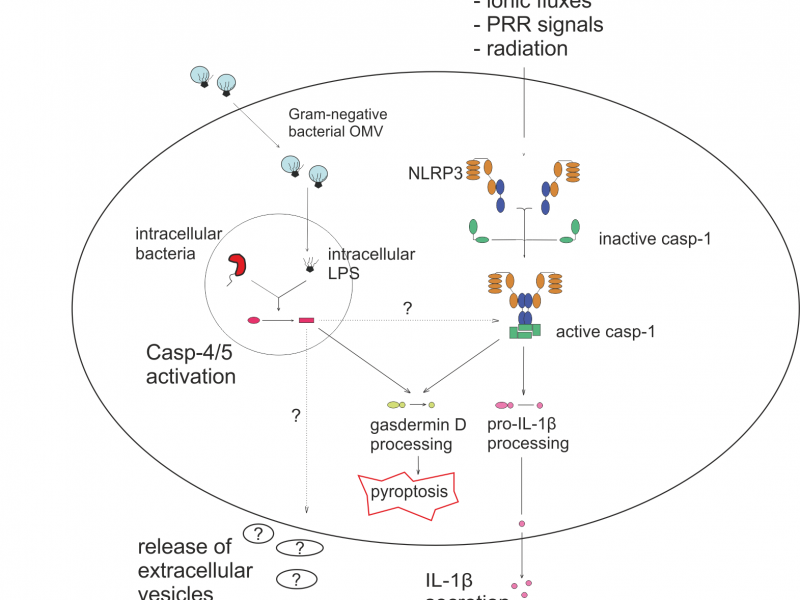 The process of secretion of extracellular vesicles, as well as the cargo they carry, is tightly regulated and dependent on the state of the immune system. Our previous studies have provided evidence that induction of inflammatory response via inflammasome has a profound impact on the secretion and cargo selection of extracellular vesicles in human macrophages. Our research in this field aims at better understanding of this apparent phenomenon of vesicular communication in inflammation, specifically by investigating the molecular links connecting the function of the NLRP3 and non-canonical caspase-4/5/11 inflammasomes and extracellular vesicle secretion.
The process of secretion of extracellular vesicles, as well as the cargo they carry, is tightly regulated and dependent on the state of the immune system. Our previous studies have provided evidence that induction of inflammatory response via inflammasome has a profound impact on the secretion and cargo selection of extracellular vesicles in human macrophages. Our research in this field aims at better understanding of this apparent phenomenon of vesicular communication in inflammation, specifically by investigating the molecular links connecting the function of the NLRP3 and non-canonical caspase-4/5/11 inflammasomes and extracellular vesicle secretion.
4. Proteomic analyses of extracellular vesicles
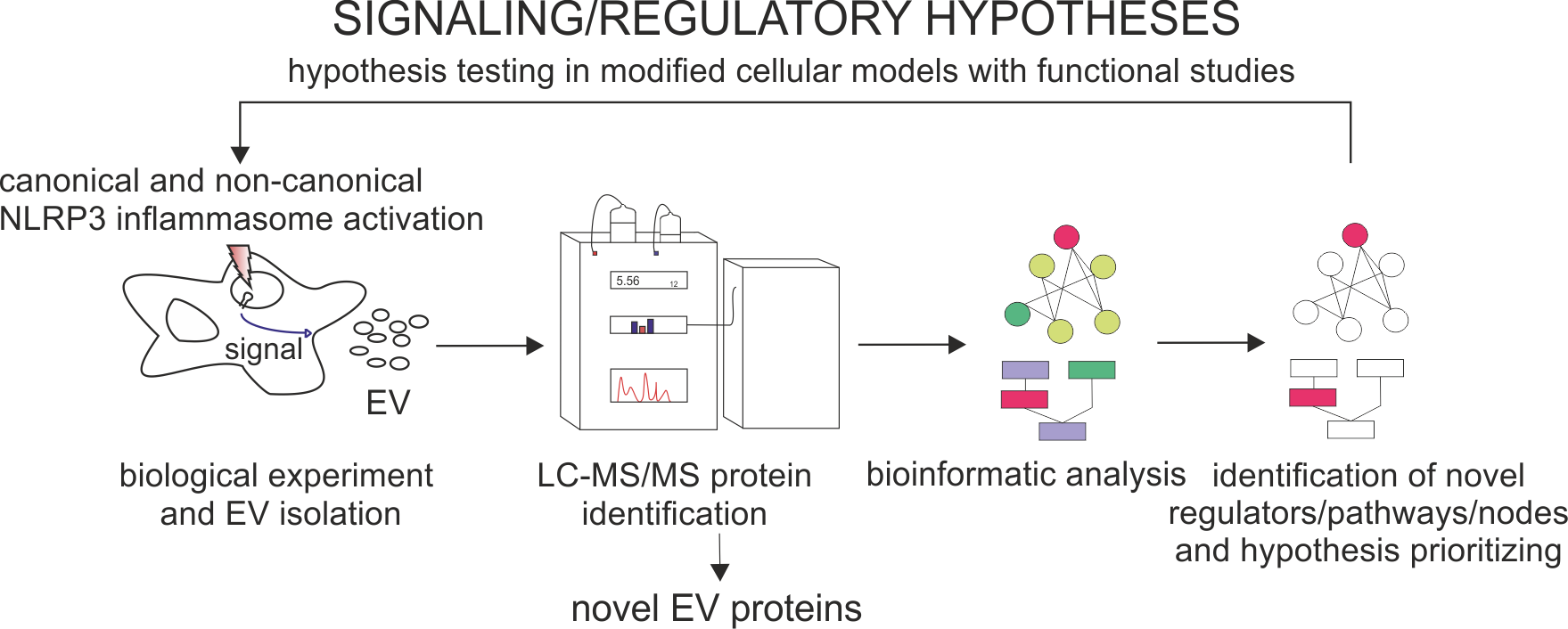 In collaboration with the Universities of Oslo and Helsinki we conduct mass spectrometry proteomics of extracellular vesicles, which upon detailed bioinformatic analyses allow to reveal novel biological roles the vesicles play in regulation of immune responses in inflammation. Profiling of the protein composition of extracellular vesicles may also be a valuable source of information for investigations of signaling networks involved in inflammatory response, as well as in predicting the biological meaning of extracellular vesicle delivery from one cell to another.
In collaboration with the Universities of Oslo and Helsinki we conduct mass spectrometry proteomics of extracellular vesicles, which upon detailed bioinformatic analyses allow to reveal novel biological roles the vesicles play in regulation of immune responses in inflammation. Profiling of the protein composition of extracellular vesicles may also be a valuable source of information for investigations of signaling networks involved in inflammatory response, as well as in predicting the biological meaning of extracellular vesicle delivery from one cell to another.
5. New methods for purification of extracellular vesicle populations.
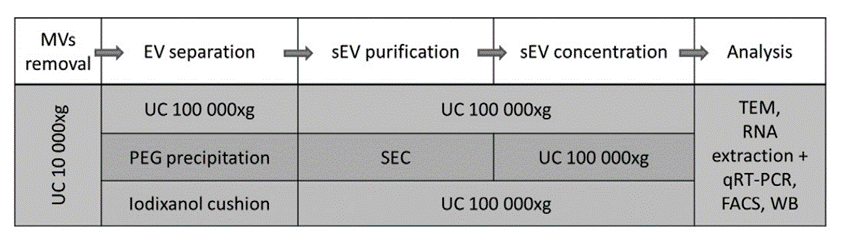
Extracellular vesicles, including exosomes, are isolated from biological fluids – both obtained from human body and in vitro cell cultures. However, the separation of various populations of vesicles (e.g. small exosomes from larger microvesicles) is still a major challenge hampering the analysis. New methods allowing for selective enrichment or isolation of particular populations are highly desired if the vesicles are to be used in therapy. Our team is also involved in studies aiming at designing optimal methods for extracellular vesicle purification, by optimization of known methods of vesicle isolation: ultracentrifugation, density gradients, chemical precipitation and size exclusion chromatography. To verify the purity of obtained populations we use electron microscopy and biochemical methods: RNA composition, in-gel analyses and flow cytometry.
6. Modulation of exosome production to support anti-cancer treatment
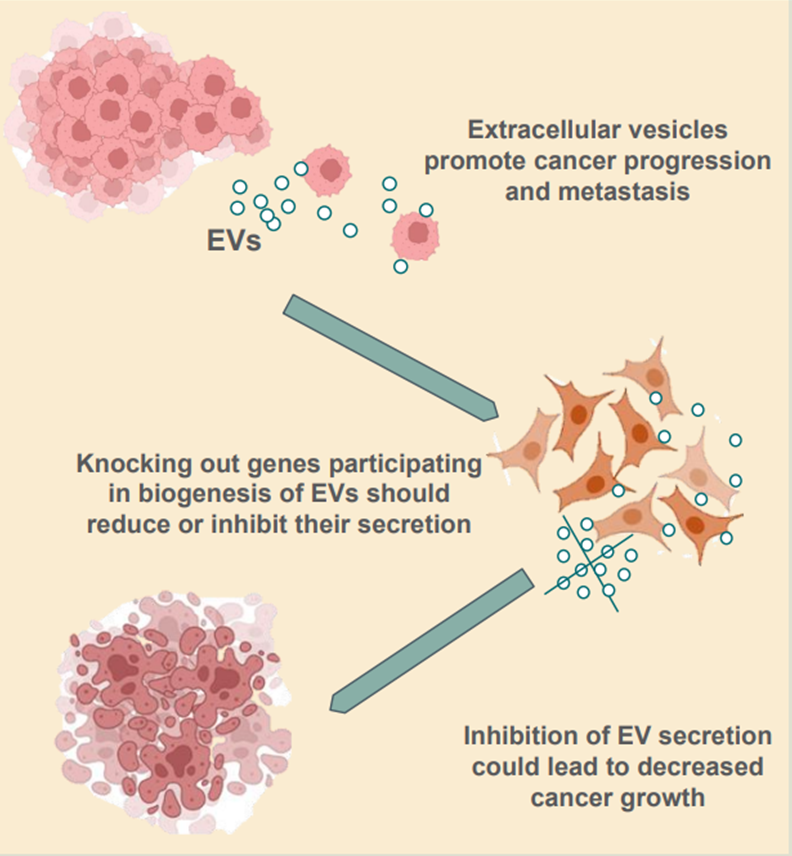
In the human body, potential cancer cells arise every day. Only when a cancer cell is able to escape and overcome the immune response it can start to grow. Thus, cancer cells have to communicate intensively with other body cells to inhibit immune destruction and to organize adequate supply. Exosomes help to shut down anti-cancer immune responses and to induce the growth of new blood vessels. They support the survival of cancer cells in many different ways. For example, exosomes export, from the cell to the outside, substances harmful to the cancer cell, such as cytotoxic drugs used in chemotherapy. In some cancer patients, the disruption of the anti-tumor immune response may be entirely dependent on exosome activity. Thus, blocking the exosome production in cancer cells is expected to facilitate anti-cancer therapies or even induce immunity. The main goal of the proposed project is to characterize melanoma cells in vitro in which the production and secretion of exosomes was disabled to explore the feasibility of such a therapeutic approach.
Cypryk W.; Czernek L.; Horodecka K.; Chrzanowski J.; Stańczak M.; Nurmi K.; Bilicka M.; Gadzinowski M.; Walczak-Drzewiecka A.; Stensland M.; Eklund K.; Fendler W.; Nyman T., and Matikainen S. Lipopolysaccharide Primes Human Macrophages for Noncanonical Inflammasome-Induced Extracellular Vesicle Secretion. Journal of Immunology. 2023, 210:(3):322-334
Czernek L., Pęczek Ł. & Düchler M. Small Extracellular Vesicles Loaded with Immunosuppressive miRNAs Leads to an Inhibition of Dendritic Cell Maturation. Arch. Immunol. Ther. Exp. 2022, 70, 27
Kluszczyńska K, Pęczek Ł, Różański A, Czernek L, Duechler M. U6/miR-211 expression ratio as a purity parameter for HEK293 cell-derived exosomes. Acta Biochim Pol. 2022, 69(2):409-415
Horodecka K, Düchler M. CRISPR/Cas9: Principle, Applications, and Delivery through Extracellular Vesicles. Int J Mol Sci. 2021, 22(11):6072.
Brambilla D, Sola L, Ferretti AM, Chiodi E, Zarovni N, Fortunato D, Criscuoli M, Dolo V, Giusti I, Murdica V, Kluszczyńska K, Czernek L, Düchler M, Vago R, Chiari M. EV Separation: Release of Intact Extracellular Vesicles Immunocaptured on Magnetic Particles. Anal Chem. 2021, 93(13):5476-5483
Exosomes as messengers between mother and fetus in pregnancy. Int J Mol Sci. 2020; 21 (12): E4264.
Kluszczyńska K, Czernek L, Cypryk W, Pęczek Ł, Düchler M. Methods for the determination of the purity of exosomes. Curr Pharm Des. 2019; 25 (42): 4464-85.
Düchler M, Czernek L, Peczek L, Cypryk W, Sztiller-Sikorska M, Czyz M. Melanoma-derived extracellular vesicles bear the potential for the induction of antigen-specific tolerance. Cells. 2019 Jul 2; 8(7). pii: E665.
Düchler M. Int”Dll”igent control of T-cell pathology in GVHD. Blood. 2018 Nov 15; 132(20):2112-2114.
Cypryk W, Nyman TA, Matikainen S. From inflammasome to exosome – does extracellular vesicle secretion constitute an inflammasome-dependent immune response? Front Immunol. 2018 Sep 25; 9:2188.
Nyman TA, Lorey MB, Cypryk W, Matikainen S. Mass spectrometry-based proteomic exploration of the human immune system: focus on the inflammasome, global protein secretion, and T cells. Expert Rev Proteomics. 2017 May; 14(5):395-407.
Wozniak M, Peczek L, Czernek L, Düchler M. Analysis of the miRNA profiles of melanoma exosomes derived under normoxic and hypoxic culture conditions. Anticancer Res. 2017 Dec; 37(12):6779-6789.
Czernek L, Düchler M. Functions of cancer-derived extracellular vesicles in immunosuppression. Arch Immunol Ther Exp (Warsz). 2017 Aug; 65(4):311-323.
Düchler M, Leszczyńska G, Sochacka E, Nawrot B. Nucleoside modifications in the regulation of gene expression: focus on tRNA. Cell Mol Life Sci. 2016 Aug; 73(16):3075-95.
Czernek L, Chworos A, Düchler M. The uptake of extracellular vesicles is affected by the differentiation status of myeloid cells. Scand J Immunol. 2015 Dec; 82(6):506-14.
Düchler M, Peczek L, Szubert M, Suzin J. Influence of hypoxia inducible factors on the immune microenvironment in ovarian cancer. Anticancer Res. 2014 Jun; 34(6):2811-9.
Gajos-Michniewicz A, Düchler M, Czyz M. MiRNA in melanoma-derived exosomes. Cancer Lett. 2014 May 28; 347(1):29-37.
Düchler M, Peczek L, Zuk K, Zalesna I, Jeziorski A, Czyz M. The heterogeneous immune microenvironment in breast cancer is affected by hypoxia-related genes. Immunobiology. 2014 Feb; 219(2):158-65.