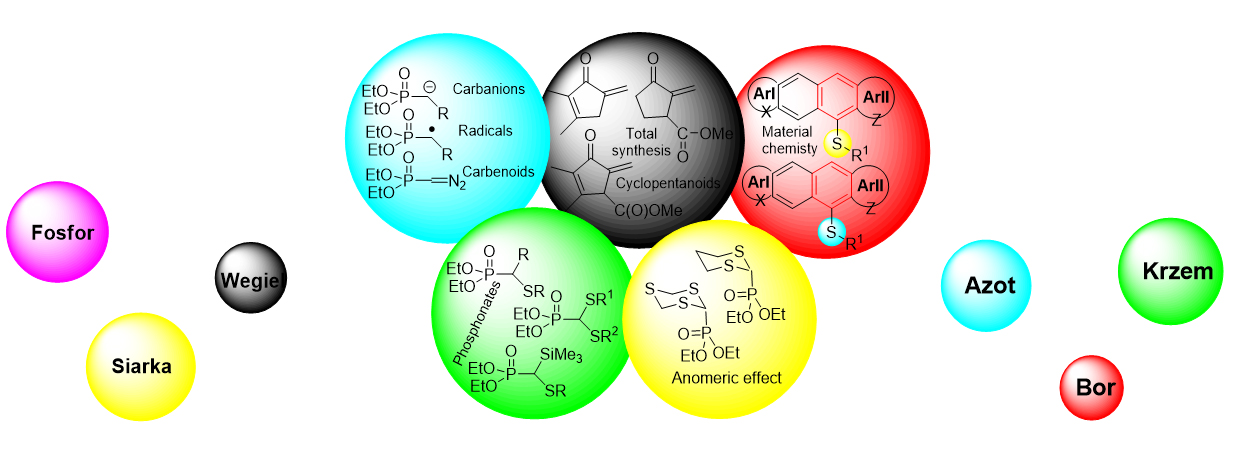
Research interests:
- organic and heteroorganic chemistry (P, S, N, Si, B);
- new concepts in organic chemistry;
- chemistry of materials (synthesis and properties of optoelectronic materials based on small organic molecules);
- synthesis of natural products and biologically active compounds (cyclopentanoids, lignanoids, alkaloids, biologically active quaternary heteronium salts, including optically active ionic liquids, bio-dendrimers);
- studies on reactions involving reactive intermediates (carbocations – SEAr, carbanions, carbon-centered radicals at the phosphorus atom; reaction mechanisms);
- sonochemistry;
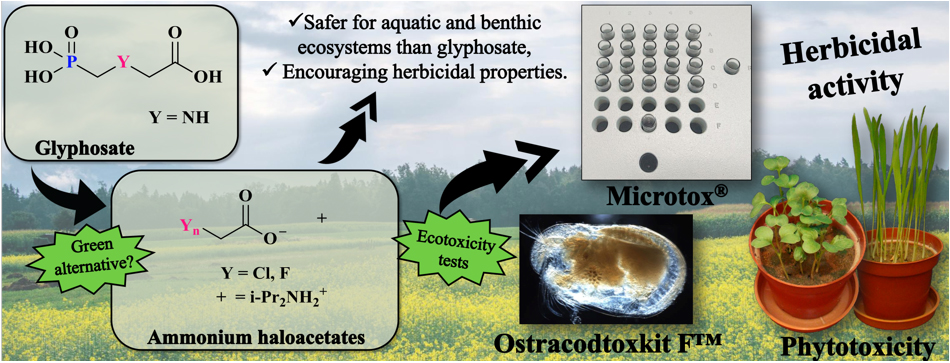
- ecotoxicological and agricultural chemistry (studies on the eco(phyto)toxic properties of organic compounds, including drugs as well as studies on herbicidal properties of new heteroorganic compounds,
- pharmaceutical chemistry (improvement of bioavailability of antihypertensive drugs, drugs against COVID 19).
The studies are carried out in cooperation with the Structural and Material Research Laboratory (PB) at the JDU, in Częstochowa.
Antiviral polyanionic dendrimer (Na-salt) against both types of HIV
Recent scientific achievements:
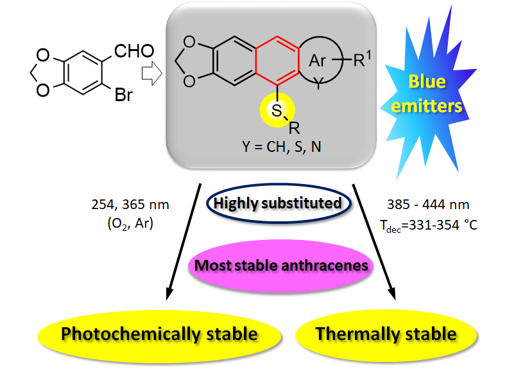
1. Discovery of two, new hetero variants of the-Friedel-Crafts/Bradsher reaction, as an oxygen (oxo-F-C/B)1-6 and sulfur variant (thio-F-C/B).6,7
Meaning: These reactions lead to the formation of unique RO- and RS-substituted (hetero) acenes characterized by: 1) high degree of substitution, 2) presence of at least three fused (hetero)aromatic rings and 3) electron-donor or electron donor-acceptor character which distinguishes the acenes in terms of chemical structure and unique properties from other known (hetero) acenes.
Literature:
- Diarylmethanol Derivative into an Unknown 1,2,3,6,7,10-Hexahydroxylated Anthracene System. P. Bałczewski, M. Koprowski, A. Bodzioch, B. Marciniak, E. Różycka-Sokołowska, J. Org. Chem. 2006, 71, 2899-2902.
- First Approach to Nitrogen‐Containing Fused Aromatic Hydrocarbons as Targets for Organoelectronics Utilizing a New Transformation of O‐Protected Diaryl Methanols. P. Bałczewski, A. Bodzioch, E. Różycka-Sokołowska B. Marciniak, P. Uznański, Chem. Eur. J. 2010,16, 2392-2400.
- Synthesis and Optoelectronic Properties of Hexahydroxylated 10‐O‐R‐Substituted Anthracenes via a New Modification of the Friedel–Crafts Reaction Using O‐Protected ortho‐Acetal Diarylmethanols. A Bodzioch, B. Marciniak, E. Różycka-Sokołowska, J. K. Jeszka, P. Uznański, S. Kania, J. Kuliński, P. Bałczewski, Chem. Eur. J. 2012, 18, 4866-4876.
- Milder Bradsher Conditions for Blue light Emitting Anthracenes. A. Bodzioch, B. Marciniak, E. Różycka-Sokołowska, J. K. Jeszka, P. Uznański, S. Kania, J. Kuliński, P. Bałczewski, Synfacts – Highlights in Curr. Synth. Org. Chem. 2012, 8, 619-625.
- Use of Isomeric, Aromatic Dialdehydes in the Synthesis of Photoactive, Positional Isomers of Higher Analogs of o-Bromo(hetero)acenaldehydes. P. Bałczewski, J. Skalik, P. Uznański, D. Guziejewski, W. Ciesielski, RSC Adv. 2015, 5, 24700-24704.
- Ultrasound-assisted synthesis of RO- and RS-substituted (hetero)acenes via oxo- and thio-Friedel-Crafts/Bradsher reactions, P. Bałczewski; E. Kowalska, J. Skalik, M. Koprowski, K. Owsianik, E. Różycka-Sokołowska, Ultrason. Sonochem. 2019. https://doi.org/10.1016/j.ultsonch.2019.104640.
- Mono-aryl/alkylthio-substituted (Hetero)acenes of Exceptional Thermal and Photochemical Stability via Thio-Friedel Crafts/Bradsher Cyclization Reaction. P Bałczewski, E. Kowalska, E. Różycka-Sokołowska, J. Skalik, K. Owsianik, M. Koprowski, B. Marciniak, D. Guziejewski, W. Ciesielski, Chem. Eur. J. 2019, https://onlinelibrary.wiley.com/doi/10.1002/chem.201903027
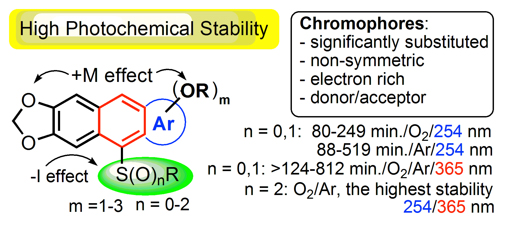
2. Discovery of new organic materials with very high thermal and photochemical resistance.1
Meaning: The highly substituted RS-(hetero)acenes obtained in the oxo-FC/B and thio-FC/B reactions have very high thermal (Tdec. = 331-354 oC) and exceptional photochemical stability under aerobic and anaerobic conditions, both at 254 nm and 365 nm, many times exceeding the stability of known analogues and unsubstituted anthracene (up to 1200-1900 times).
Application: optoelectronics and organo-photo-catalysis.
Literature:
- Mono-aryl/alkylthio-substituted (Hetero)acenes of Exceptional Thermal and Photochemical Stability via Thio-Friedel Crafts/Bradsher Cyclization Reaction. P. Bałczewski, E. Kowalska, E. Różycka-Sokołowska, J. Skalik, K. Owsianik, M. Koprowski, B. Marciniak, D. Guziejewski, W. Ciesielski, Chem. Eur. J. 2019. https://onlinelibrary.wiley.com/doi/10.1002/chem.201903027
3. Discovery of the possibility of significant acceleration of homogeneous reactions proceeding through the ionic (carbocation) mechanism by using ultrasound sonication.1,2
Meaning: Homogeneous reactions proceeding through the SEAr carbocation mechanism, in particular oxo- and thio-F-C/B variants as well as the classic Bradsher reaction, can be, contrary to the rules of sonochemistry, significantly accelerated by means of ultrasound sonication. The unexpected acceleration compared to the reactions carried out under silent conditions exceeded 7,500 times for the oxo-variant. The mechanism involving disturbing of solvation layers and formation of the very reactive (“naked”) carbocations upon operation of the shock wave produced by the bubble collapse was considered to be the reason for this acceleration.
Literature:
- Ultrasound-assisted synthesis of RO- and RS-substituted (hetero)acenes via oxo- and thio-Friedel-Crafts/Bradsher reactions. P. Bałczewski, E. Kowalska, J. Skalik, M. Koprowski, K. Owsianik, E. Różycka-Sokołowska, Ultrason. Sonochem. 2019. https://doi.org/10.1016/j.ultsonch.2019.104640.
- Ultrasound-assisted Bradsher reaction in aqueous and non-aqueous media: First use of ultrasounds in electrophilic aromatic cyclisation leading to polyacenes. E. Kowalska, P. Bałczewski, Ultrason. Sonochem. 2017, 34, 743-753.
4. Discovery of the new pharmaceutical solid dyspersions of high bioavailability against hypertension and COVID-19
Patents for achievements 1-4:
- Aromatyczne sulfotlenki i sulfony, sposób ich wytwarzania i zastosowanie (Aromatic sulfoxides and sulfones, a method of their preparation and application). P. Bałczewski, E. Kowalska, J. Skalik, M. Koprowski, K. Owsianik, E. Różycka-Sokołowska; patent RP appl., P.429272, 14.03.2019.
- Tio-funkcjonalizowane aceny i ich zastosowanie (Thio-functionalized acenes and their application).
P. Bałczewski, , E. Kowalska, J. Skalik, M. Koprowski, K. Owsianik, E. Różycka-Sokołowska; patent RP appl., P.4292239, 12.03.2019.
- 10-Thiosubstituted pentahydroxyanthracene derivatives, a method of their preparation and intermediate compounds. P. Bałczewski, E. Kowalska, J. Skalik; eur. patent app., Appl. No EP-14460003.8, 06.02.2014; Patent RP udzielony No 28896539 2017, 01.02.2017.
- Nowe skondensowane węglowodory poliaromatyczne i poliheteroaromatyczne, sposób ich wytwarzania oraz związki pośrednie (dwa patenty o tym samym tytule). P. Bałczewski, A. Bodzioch, J. Skalik, M. Koprowski; patent PL 219155, 24.07.2014; patent PL 219334, 25.08.2014.





